Since March of 2020, our lives have been put on hold due to the Coronavirus (COVID-19). As of December 3rd, the U.S. alone has registered 14 million positive cases. (1)
Since March of 2020, our lives have been put on hold due to the Coronavirus (COVID-19). As of December 3rd, the U.S. alone has registered 14 million positive cases. (1)
The COVID-19 vaccine rolled out to the United Kingdom on December 8th, 2020, but has not been approved yet in the United States. What Point of Dispensing (POD) plans has your healthcare facility made to help vaccinate approximately 330 million people?
Key things to think about while reading this article:
Do you have the refrigeration space to keep the vaccinations at their correct temperature?
Do you have a floor plan in place to maximize the flow of patients?
Do you know how many people you will be serving? Just healthcare workers, or their families as well?
As we think about how wonderful our lives will be going back to “normal,” there are real concerns regarding the distribution of our awaited COVID-19 vaccine that could put you and your workers at risk.
First, let us start with some facts.
According to the FDA commissioner and ABC News, the “FDA [is] working day and night on COVID-19 vaccine emergency use authorization.” (2) They use their limited time to evaluate and clear multiple coronavirus vaccines before being distributed to the public.
There is a third vaccine by AstraZeneca, but their data and vaccine dosage has some irregularities, so that we will be focusing on the Pfizer/BioNtech and Moderna vaccines. (9)
How do the vaccines work?
Pfizer/BioNtech and Moderna have used a similar approach. They have both used messenger RNA technology to snip “the virus’s genetic code to instruct cells to build the spike protein on the surface of the coronavirus, teaching the immune system to recognize the real thing.” (3)
So, what exactly is taking so long?
A typical approval timeline for vaccine clinical trials–for Pfizer (44,000 people) and Moderna (30,000 people)—would typically take up between “three or four months, maybe even longer.” (2)
FDA Commissioner, Dr. Stephen Hahn, told ABC News that the FDA is trying to get the process completed in about three weeks.
“The FDA is looking at the manufacturing data, specifically the quality around manufacturing the clinical data that show whether the vaccine is safe and effective, and the data surrounding adverse effects.”
Who will get the vaccines once the FDA approves them?
Before you get too excited about getting the vaccine for yourself, the CDC advisory group has said that healthcare workers and nursing home residents should be the first to get the vaccine. (4)
The Washington Post stated that “The recommendation to prioritize healthcare workers and residents of long-term-care facilities in the first phase of immunization leave states and local jurisdictions- even individual medical providers- with considerable leeway to define that category and create priorities within.” (5)
Although states are not required to follow the CDC’s guidance, it gives a framework if states choose to adopt it.
In addition to scientific data, these four ethical principles will assist the Advisory Committee on Immunization Practices (ACIP) in formulating recommendations for the allocation of the COVID-19 vaccine: (6)
- Maximizing benefits and minimizing harms
- Promoting justice
- Mitigating health inequities
- Promoting transparency
The allocation of the COVID-19 vaccines “should maximize the benefits of vaccination to both individual recipients and population overall.” Which, in this case, means healthcare workers and nursing home residents.
These residents and employees of long-term-care facilities are prioritized because they account for nearly 40 percent of deaths from COVID-19.
How are the vaccines being allocated from state to state?
According to a November 24th article by National Public Radio (NPR), “Once a COVID-19 vaccine is authorized by the [FDA], allocations will be made based on the total number of adults in the state.” (10)
Alex Azar, the secretary of Health and Human Services, echoed this statement during a media briefing. Siting that allocating the vaccine based on a state’s total population would be “the most consistent and fair approach.”
What are the numbers?
According to The Washington Post, “U.S. officials anticipate having about 40 million doses of vaccines from Pfizer/BioNtech and Moderna by the end of the year, enough to immunize 20 million people.” That is just a fraction of the 330 million people located in the U.S. alone.
“Operation Warp Speed, the administration’s initiative to speed vaccine and therapeutics development, plans to send the first batch of 6.4 million doses of Pfizer’s vaccine to communities nationwide within 24 hours of FDA clearance.”
Since there will be a limited number of vaccines after FDA approval, healthcare systems will prioritize who will receive the vaccine even further. Staggering vaccinations by units, like emergency departments, give the first doses to those who have direct patient contact or those who handle infectious materials.
What does that mean overall?
The CDC’s advisory group is “prioritizing healthcare personnel because they are the people who keep the ‘machinery’ of the hospitals and the rest of the healthcare system running” for Phase 1a.
The advisory group has also expressed their support to vaccinate essential workers, which make up about 87 million people, not including healthcare workers, in Phase 1b. People 65 and older, 53 million people, and adults with a higher risk of contracting COVID-19, 100 million people, would be in Phase 1c.
What is the timeline for the vaccinations?
Based on Texas Health and Human Services as of October 19th, 2020: (7)
Phase 1: Between November and December 2020 (Now estimating between December 2020 and January 2021). As stated before, since there will be a limited supply of the vaccine, only those in the healthcare industry would receive the vaccine during this distribution.
Phase 2: Between January 2021 and July 2021 (Now estimating between February 2021 and August 2021). By this point, there is a hope to have an increased number of vaccine doses available. During this phase, the priority would fall to those left in Phase 1, and then it would move to populations that have yet to be determined by the CDC.
Phase 3: Between July 2021 and October 2021 (Now estimating between August 2021 and November 2021). There should now be a sufficient supply of a vaccine dose for the entire population during this phase.
Phase 4: Between October 2021 and beyond (Now estimating between November 2021 and beyond). There should be a sufficient supply of the vaccine due to decreased need. Most of the population should already be vaccinated; anyone who has not received the vaccine can do so.
But hold on—how many required doses do we need?
Here comes the tricky part.
Both Pfizer/BioNTech and Moderna require patients to have two doses of their vaccine. Pfizer requires the second dose to be administered 21 days (3 weeks) after the initial dose and will last up to 16 years. On the other hand, Moderna requires their second dose to be given 28 days (4 weeks) after the initial vaccination and will last up to 18 years.
Not only will healthcare facilities need to deal with the initial wave of those who can receive the vaccine. They will then need to keep track of who receives their vaccine and when they need to come back for their second.
How will the vaccines be shipped and stored?
Moderna’s vaccine must be frozen at minus-20 degrees Celsius (minus-4 degrees Fahrenheit), but it will keep for a month at refrigerator temperatures.
“This could make it easier to distribute to pharmacies and rural areas that don’t have specialized freezers.” (3)
Pfizer/BioNTech’s vaccine must be kept at an ultracold, minus-70 degrees Celsius (minus-94 degrees Fahrenheit).
“The company created its own GPS-tracked coolers filled with dry ice to distribute it.” Each vial of the Pfizer vaccine holds five doses when diluted. Once thawed, the undiluted vial can be kept in a refrigerator for only five days. A diluted vial can be kept for only six hours before it must be discarded.” (3)
What about rural states?
Getting the vaccine to rural areas of the country may prove difficult with the added cold storage requirements. Many states “lack the adequate infrastructure to ensure the safe and effective delivery of COVID-19 vaccines that will require special refrigeration.” (11)
Molly Howell, North Dakota immunization program manager at the Department of Health, told CNBC in October that “We have procured some ultra-cold chain freezers and transport freezers. Dry ice is still on the table and in the works. Because we’re such a rural state, we want to make sure we’re able to get it to the rural areas.”
The U.K. has already started giving out the vaccine.
Margaret Keenan became the first person vaccinated against COVID-19 on December 8th, 2020, in the United Kingdom. The 91-year-old grandmother of four said, “I can finally look forward to spending time with my family and friends in the new year after being on my own for most [of] the year.” (12)
In a plan similar to what the CDC advisory committee has provided, the U.K. will vaccinate front-line health workers, long-term care facility workers, and residents over 80 years old, first. After that, the vaccines will be made available at hospitals before being given to doctor’s offices to distribute.
England’s National Health Service chief executive, Sir Simon Stevens, told NPR, “It will take some months to complete the work as more vaccine supplies become available, and until then, we must not drop our guard. But if we all stay vigilant in the weeks and months ahead, we will be able to look back at this decisive turning point in the battle against the virus.” (12)
What does this all mean?
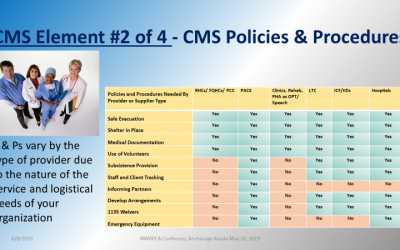
Here are vital questions you should be asking as a healthcare facility (8)
Healthcare facilities must have a Point of Dispensing (POD) plan for vaccine distribution to work effectively and seamlessly.
- Who are your key people? Do they include: Human Resource Personnel, Managers, Medical Advisors, Logistics Specialists, and Security Staff?
- How many people should you expect to serve?
- Will your POD be open to just staff, or staff and their families?
- Where should your POD be located?
- Is there a separate entrance and exit?
- Can it be easily located and accessible?
- Will your dispensing area be able to accommodate tables, chairs, and many people while still meeting social distancing regulations?
- Is there a place to secure medications and supplies?
- Will your POD location be large enough to be a drive-through versus a walk-through facility?
- Do you have a design/layout of your POD site?
- Will your layout impact the efficiency of POD operations?
- Do you have a floor plan to maximize the flow of POD participants?
Sources for article:
- https://www.nytimes.com/interactive/2020/us/coronavirus-us-cases.html
- https://abcnews.go.com/Politics/fda-working-day-night-covid-19-vaccine-emergency/story?id=74491765
- https://www.washingtonpost.com/health/2020/11/17/covid-vaccines-what-you-need-to-know/?arc404=true
- https://www.cnbc.com/2020/11/30/covid-vaccine-cdc-panel-set-tuesday-to-vote-who-gets-vaccine-first.html
- https://www.washingtonpost.com/health/2020/12/01/vaccine-priority-groups-covid/
- https://www.cdc.gov/mmwr/volumes/69/wr/mm6947e3.htm#:~:text=In%20addition%20to%20scientific%20data,%3B%20and%204)%20promoting%20transparency
- https://dshs.texas.gov/uploadedFiles/Content/Consumer_and_External_Affairs/TaskForceID/docs/6_COVID-19_Vaccination_Plan%2010-19-2020.pdf
- https://www.washoecounty.us/health/programs-and-services/ephp/emergency-preparedness/point-of-dispensing/tools-for-pod-activation/pod-trainings.php
- https://www.nytimes.com/2020/11/25/business/coronavirus-vaccine-astrazeneca-oxford.html
- https://www.npr.org/sections/health-shots/2020/11/24/938617836/initial-batch-of-covid-19-vaccines-will-go-to-states-based-on-population-not-ris
- https://www.cnbc.com/2020/10/16/states-face-friday-deadline-to-submit-covid-19-vaccine-distribution-plans.html
- https://www.npr.org/sections/coronavirus-live-updates/2020/12/08/944125280/u-k-begins-nationwide-coronavirus-immunization-largest-in-nations-history